A Summary of (EU) 2017/745 and (EU) 2017/746 - A Must Read for Medical Device Manufactures
If your Medical Device Company wants to gain and maintain access to one of the largest medical devices markets, the European Economic Area (EEA) it is important to stay on the top of the regulatory changes to ensure certification of your products in a timely manner. This article presents the new rules MDR (EU) 2017/745 (MD/AIMD) and MDR (EU) 2017/746 (IVD) in a nutshell.

The Background
The medical device industry in Europe has undergone significant changes with the introduction of two pivotal regulations: the Medical Device Regulation (MDR) (EU) 2017/745 and the In Vitro Diagnostic Medical Device Regulation (IVDR) (EU) 2017/746. These regulations, adopted by the European Union (EU) to replace the previous directives, aim to improve the safety, quality, and performance of medical devices and in vitro diagnostic (IVD) devices. This comprehensive article delves into the details of MDR and IVDR, outlining the key requirements, changes from previous directives, and the implications for medical device manufacturers.
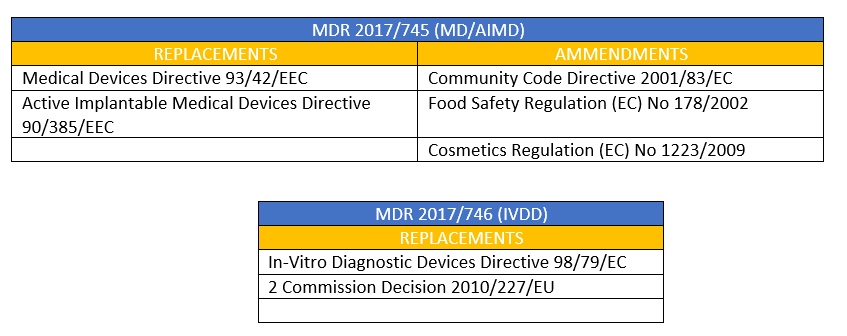
The new rules MDR (EU) 2017/745 (MD/AIMD) and MDR (EU) 2017/746 (IVD) took their form - to a large extent - from the existing three important directives which were familiarized over the past 20 years.
- Medical Devices Directive (MDD) 93/42/EEC
- Active Implantable Medical Devices Directive (AIMDD) 90/385/EEC
- In Vitro Diagnostic Devices Directive (IVDD) 98/79/EC
These directives established the framework for the safety and performance of medical devices and IVDs in the EU. However, several factors prompted the EU to revise and update these regulations:
- Technological Advancements: The medical device industry has seen rapid technological advancements, including the development of software as a medical device (SaMD), personalized medicine, and combination products. The existing directives were not fully equipped to address the complexities of these innovations.
- Patient Safety: High-profile incidents, such as the PIP breast implant scandal, highlighted gaps in the regulatory framework that allowed unsafe products to reach the market. Strengthening patient safety became a priority.
- Regulatory Consistency: The previous directives allowed for varying interpretations by member states, leading to inconsistencies in the application of regulations across the EU. A harmonized approach was needed to ensure a level playing field for manufacturers and to enhance market surveillance.
Key Changes
- stricter ex-ante control for high-risk devices via a new pre-market scrutiny mechanism with the involvement of a pool of experts at EU level.
- the reinforcement of the criteria for designation and processes for oversight of Notified Bodies.
- the inclusion of certain aesthetic devices which present the same characteristics and risk profile as analogous medical devices under the scope of these Regulations.
- improved transparency through the establishment of a comprehensive EU database on medical devices and of a device traceability system based on Unique Device Identification;
- the introduction of an "implant card" containing information about implanted medical devices for a patient.
- the reinforcement of the rules on clinical evidence, including an EU-wide coordinated procedure for authorisation of multi-centre clinical investigations.
- the strengthening of post-market surveillance requirements for manufacturers.
- improved coordination mechanisms between EU countries in the fields of vigilance and market surveillance.
MDR (EU) 2017/745 (MD/AIMD) and MDR (EU) 2017/746 (IVD) encourage a much more robust product-life cycle approach with an emphasis on proactively managing device safety and performance. However, the new regulations also recognize how the advancements in technology, continuous product design innovations, and the changing healthcare systems delivered over the last twenty years have left an impact. In general, the new rules are a breakthrough to the progress of the single market strategy and accomplishing a striking a balance between the regulatory concerns and economic opportunities as envisioned by the European Union.
Replacements and Amendments
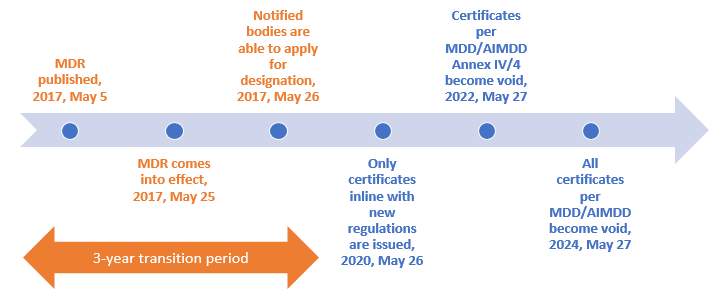
Transition timeline for (EU) 2017/745 (MDR & AIMDR)
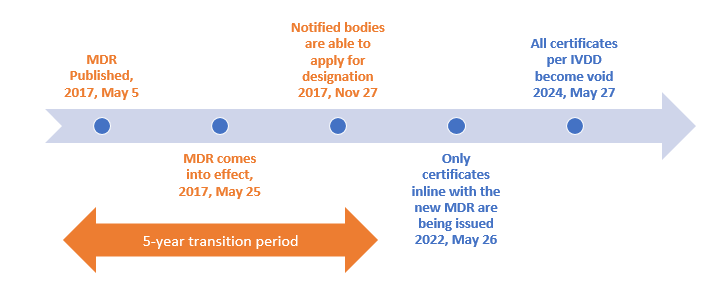
Transition timeline for MDR (EU) 2017/746 (IVDR)
How can medical device manufactures prepare for the transition?
As a lot of work must be accomplished in a short time of the transition period, you need to get ready now to ensure your products are compliant. Here's how you can prepare:
- Ensure that all relevant professionals at your company have a thorough understanding of the new regulation including key changes.
- Perform a thorough gap analysis to review your existing products with the new regulation. In the gap analysis, also consider the reclassification of some product groups and the MDRs description of a medical device.
- Attend relevant training programs for compliance with the medical device regulations.
Implications for Medical Device Manufacturers
The introduction of MDR and IVDR has significant implications for medical device and IVD manufacturers. Compliance with these regulations is mandatory for companies that wish to market their products in the EU. Below are some key considerations for manufacturers:
Increased Regulatory Burden
MDR and IVDR introduce more stringent requirements for clinical evaluation, performance evaluation, conformity assessment, and post-market surveillance. Manufacturers must invest in additional resources, including personnel, training, and technology, to meet these requirements. This increased regulatory burden can be particularly challenging for small and medium-sized enterprises (SMEs).Re-Evaluation of Product Portfolios
Manufacturers must re-evaluate their product portfolios to ensure compliance with the new classification rules and conformity assessment procedures. This may involve reclassifying devices, conducting new clinical or performance studies, and updating technical documentation. In some cases, manufacturers may decide to discontinue certain products if the cost of compliance is too high.Collaboration with Notified Bodies
The role of Notified Bodies has been significantly enhanced under MDR and IVDR, with more stringent criteria for their designation and oversight. Manufacturers must establish strong relationships with Notified Bodies and engage with them early in the conformity assessment process. Delays in the availability of Notified Bodies have been a concern, making early engagement even more critical.Supply Chain Management
Manufacturers must ensure that their suppliers and subcontractors comply with MDR and IVDR requirements, particularly regarding material sourcing, testing, and documentation. This may involve renegotiating contracts, conducting supplier audits, and implementing robust quality management systems.Post-Market Surveillance and Vigilance
MDR and IVDR place a greater emphasis on post-market surveillance and vigilance, requiring manufacturers to actively monitor the performance of their devices and respond to incidents promptly. Manufacturers must establish and maintain comprehensive PMS and vigilance systems, including data collection, analysis, and reporting mechanisms.Transition Planning
The transition periods for MDR and IVDR have given manufacturers some time to comply with the new regulations. However, the transition is complex and requires careful planning. Manufacturers must develop and implement transition plans, including timelines, resource allocation, and risk management strategies, to ensure a smooth transition to the new regulatory framework.
Supplementary information for manufacturers interested in selling their medical devices in the European Union:
To sell their devices in the European markets Manufacturers must meet the Medical Device Directives' quality standards. The EN ISO 13485 meets most of the QMS requirements outlined in the Directives. Many successful medical device companies choose to implement it prior to entering the EU medical device markets. Browse through medical device training programs and choose a relevant training program that provides a thorough understanding into its implementation.
The webinars Post Market Surveillance with the new Medical Device Regulation EU MDR 745/2017 and Supplier Management with the new Medical Device Regulation EU MDR 745/2017 provide specific supplementary information on the topic in detail.
The Conclusion
The introduction of the Medical Device Regulation (MDR) (EU) 2017/745 and the In Vitro Diagnostic Medical Device Regulation (IVDR) (EU) 2017/746 represents a significant shift in the regulatory landscape for medical devices and IVDs in the EU. These regulations aim to enhance patient safety, improve device quality and performance, and ensure regulatory consistency across the EU.
For medical device manufacturers, compliance with MDR and IVDR is mandatory and presents both challenges and opportunities. The increased regulatory burden requires manufacturers to invest in resources, re-evaluate product portfolios, and collaborate closely with Notified Bodies. However, successful compliance can lead to improved product safety, market access, and brand reputation.
As the medical device industry continues to evolve, manufacturers must stay informed about regulatory developments, engage with stakeholders, and implement effective compliance strategies. By doing so, they can navigate the complexities of MDR and IVDR and contribute to a safer, more innovative healthcare environment in the EU.