How to Develop Effective training programs for FDA Compliance
One of the frequently cited deviations in FDA inspectional observations and warning letters is the lack of adequate training of employees. Training is an FDA requirement that should be planned, conducted, evaluated and documented. It is vital for employees of regulated companies to understand what the regulations are, their interpretations and FDA requirements.

This article is designed to help FDA regulated companies understand the GXP training requirements in US and EU and develop effective training programs and plans. For a firsthand understanding of the effective training practices for FDA compliance, attend this webinar.
We begin with the basics of understanding GXPs and then move on to the following six steps that will help you design and develop effective training programs that your organization needs:
- Define competencies for job roles
- Conduct a training needs assessment
- Source trainers
- Design and deliver learning interventions
- Assess the effectiveness of the interventions and Gather learners' feedback
- Manage the documentation
Get to know the GXPs
Before getting into the importance of GXP training, it's vital to get the context right. So, here goes its definition. GXP is an acronym commonly used in the bio/pharmaceutical industry.
These regulations which are established by the United States and Food and Drug Administration (FDA) are published in the Code of Federal Regulations.
These regulations are divided into Titles, Code of Regulation, and Sections. Whenever a regulation is cited, the title tells where the regulation is published.
Let's take 21 CFR 211.80 as an example:
It is important for personnel in the regulated organizations to read the regulations pertaining to their roles or specific area periodically even if they have read it before. To quickly find and research regulations specific to your role or area, search this website.
Some Regulations that need training as a key part of the Quality Management System
- FDA 21 CFR Part 211.25 for pharmaceutical companies
- FDA 21 CFR Part 820.25 for medical device manufacturers
- ISO 9000 2000 for general manufacturers and other businesses
- ISO 13485 for medical device manufacturers
To review types of training requirements from the FDA and ISO 13485 for medical device employee attend this webinar. It will discuss techniques for monitoring and documenting training effectiveness.
21 CFR 211.25
- (a) Each person engaged in the manufacture, processing, packing, or holding of a drug product shall have education, training, and experience, or any combination thereof, to enable that person to perform the assigned functions.
- Training shall be in the particular operations that the employee performs and in current good manufacturing practice (including the current good manufacturing practice regulations in this chapter and written procedures required by these regulations) as they relate to the employee's functions.
- Training in current good manufacturing practice shall be conducted by qualified individuals on a continuing basis and with sufficient frequency to assure that employees remain familiar with CGMP requirements applicable to them.
21 CFR 58.29
- Each individual engaged in the conduct of or responsible for the supervision of a nonclinical laboratory study shall have education, training, and experience, or combination thereof, to enable that individual to perform the assigned functions.
- Each testing facility shall maintain a current summary of training and experience and job description for each individual engaged in or supervising the conduct of a non-clinical laboratory study.
GXP Training Guidelines, benefits and contents

GXP Guidelines to develop competent individuals
To help organizations abide by the GXP regulations, the International Academy of Clinical Research (IAoCR) set up a task force to produce GXP training guidelines. These guidelines provide best training practices for those involved in the training and development of clinical research professionals and can be used by any regulated organizations irrespective of their size or sectors.
The GXP guidelines provide the Key steps in developing competent individuals and Competency framework through assessment of learning and its documentation.
Benefits of Developing Competent individuals
- Helps in protecting the rights and well-being of patients
- Helps secure verifiable and accurate data
- Cuts costs of errors and rework because of incompetence
- Faster development of new treatments
- Provides an opportunity to demonstrate a real interest in people
- Increased staff retention
- Provides a basis for promotions and succession planning
Contents of GXP guidelines
The GXP guidelines cover how to
- Define competencies for job roles
- Conduct a training needs assessment
- Source trainers
- Design and deliver learning interventions
- Assess the effectiveness of the interventions
- Gather learners' feedback
- Manage the documentation
The guidelines also provide examples, templates, and a direction to useful resources for those who may need a deeper coverage of subject matter.
How to Develop Effective Training Programs
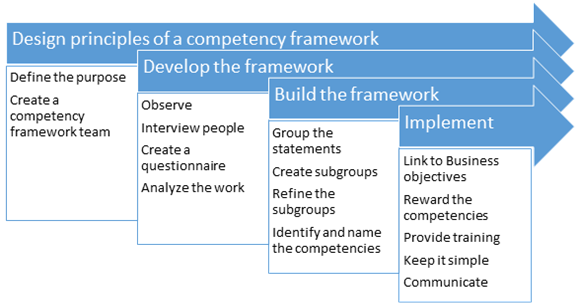
1. Build a competency framework
Going by the GXP guidelines, the first step to developing an effective training program is to define competencies for job roles. The benefits of defining competencies required for the success of your organization can help you
- Make sure that your staff demonstrate the required expertise
- Source and employ new staff more effectively
- Assess performance efficiently
- Assess skills and competency gaps effectively
- Provide more customized training and professional development
- Make effective succession plans
- Enable an efficient change management process
To define competencies for job roles, collect and combine competency information. This information will help you develop a standardized approach for performance. It more specifically outlines what is needed for people to be effective in their roles and helps them see how their roles relate to the organizational goals.
2. Conduct a training needs analysis
Four phases are recommended in conducting a training needs analysis.
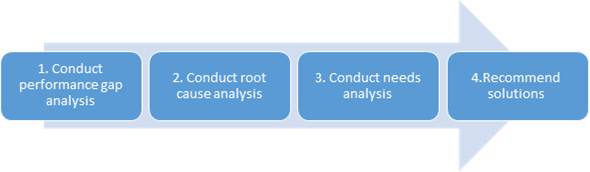
Conduct performance gap analysis
If there are discrepancies between the desired performance outcome of your business and the actual business performance, the gap between the two is called the performance gap. By comparing your current and desired business performance result, you arrive at the performance gap.
Conduct root cause analysis
Root cause analysis helps you identify the likely causes of the performance gaps. By identifying the root cause, you can think of solutions to bridge the gap.
Conduct needs analysis
Needs analysis helps you determine the types of needs to address the root cause. By performing a detailed analysis, you will be helped to design and implement a relevant intervention for a performance issue.
Recommend solutions
When performance is an ongoing issue, training is the solution. If you identify the following reasons, training can be the proposed solution.
- Inadequate knowledge or skill deficiency
- Updates in regulatory guidelines requiring new knowledge or skills
- Insufficient knowledge of new technologies
- Lack of coaching on higher performance standards
- Lack of motivation for new employees
3. Source Trainers
The two steps mentioned above will help you source trainers according to your requirements. There are advisory networks and online communities of GRC professionals and experts from which you can source trainers.
FDA regulations mandate that "Training in current good manufacturing practice shall be conducted by qualified individuals"
4. Design and deliver learning interventions, gather learner's feedback
Training is scheduled in many ways. Training that is delivered in response to a deficiency in performance or qualification and training that is delivered according to the calendar have basic distinctions.
Training that is delivered in response to deficiency in performance or qualification includes:
- New employee orientation (NEO)
- Training for business process redesign
- Training for standard operating procedure (SOP) revision
- Most technical training
Training intervention remediates lack of skill, information, or motivation.
Training according to the calendar includes
- Regulatory training
- Refresher training
By performing an implicit or explicit business risk assessment, the decisions about either of the above two kinds of training are made.
5. Assess the effectiveness of the interventions
Ways to access:
Collect data:
- What did the participant think about the training program? This can be achieved by using user-friendly forms.
- What did the participant learn? This can be achieved through a written examination to measure the transfer of knowledge. The questions for the examination must be clear and unambiguous. The questions should be an accurate reflection of the key points in the subject matter presented.
- Is the knowledge being applied to the task? This can be identified by quantifying the types of errors previously made as compared to any errors made after the training.
- How did the company benefit from the training?
- Are the errors reduced?
- Has the output of correctly manufactured product or device increased?
- Are the fewer documentation errors?
- Is there increased awareness?
- What types of improvements are proposed as an outcome of the training?
- Is the change control properly followed?
Manage the documentation
The FDA requires regulated companies to have standard operating procedures that define their training programs. Your SOPs for documenting your training program must include:
- The reason for the training
- Who is conducting the training?
- How the training is conducted (online, classroom, etc.)?
- When the training will be conducted and what is the frequency?
- Who will be the attendees (role-based programs)?
- What is the measurement process?
A good Learning Management System (LMS) Technology makes it easier for you to automate and manage your compliance training initiatives. Depending on the feature enablement, an LMS can
- Assign and track training
- Allow access to eLearning and virtual learning
- Schedule and assign instructor-led training
- Control course enrollment
- Send email notifications,
- Serve as a repository for documentation, course materials and historical training data
- Engage staff with better visibility into their own personalized training curriculums.
- Transition live training to online training reducing your overall costs for delivery
- Ensure that your training programs are understood, effective and well received
- Make training accessible across distributed workforces
- Run reports easily when you need them and with the granularity, you need across departments, business divisions, operating facilities, and more
Useful resources
Effective training program for FDA compliance
This FDA compliance webinar will help the attendees develop an effective training program and training plans for an organization. Attendees will learn GxP training requirements in US and EU.
The speaker Dr. Ludwig Huber, Ph.D., is the director of Labcompliance and editor of www.labcompliance.com, the global online resource for validation and compliance. He is the author of the books "Validation and Qualification in Analytical Laboratories" and "Validation of Computerized Analytical and Networked Systems". He has given multiple presentations mainly on GLP/GMP, 21 CFR Part 11 and Validation around the world. This included seminars, workshops and presentations for the US FDA, China SFDA, Korea MFDS, Singapore HSA, ISPE, Japan PDA, PIC/S and several other national health care agencies.
Medical device employee training - requirements and implementation tips
This webinar will review types of training requirements from the FDA and ISO 13485 for medical device employee. It will discuss techniques for monitoring and documenting training effectiveness.
The speaker Betty Lane, has over 30 years' experience in medical device quality assurance and regulatory affairs. She is the founder and President of Be Quality Associates, LLC, a consulting company helping small and medium sized medical device and diagnostic companies implement and improve their quality systems for both FDA and ISO 13485 compliance band business when they became FDA and industry requirements. Her are areas of expertise include training, auditing, supplier management, design controls, software validation and general safety.
Betty's training experience includes over 25 years of training on all areas ISO 13485 and FDA cGMP, in companies where she worked as manager or director, and for AAMI, ASQ biomedical division, and ASQ sections. She has taught courses in medical quality and regulatory affairs as an Adjunct at Northeastern University, Boston, MA. Betty is active in her local section of the American Society for Quality and is also a member of the RAPS, Association for the Advancement of Medical Instrumentation (AAMI), The Society of Women Engineers and the IEEE. Betty has degrees in engineering from Rensselaer Polytechnic Institute, and an MBA from Northeastern University.








