FDA Inspection Guide - What to do before, during, and after the FDA Inspection
What are your top concerns about FDA inspection? The first step to address concerns is to gain an understanding of how to prepare for an FDA inspection. This article provides guidance about what to do before, during and after the inspection to ensure proper planning. By developing an inspection readiness plan, you not only reduce concerns but also infuse inspector confidence in the quality system.

Pre-Inspection preparation recommendations
Developing Winning Strategies for Successful FDA Inspections are crucial during the preparation process.
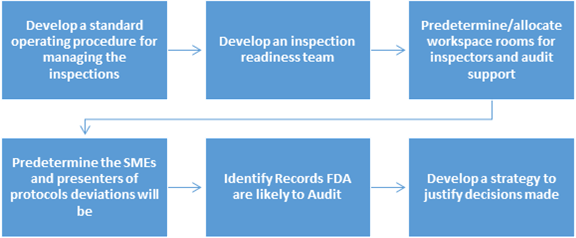
The 'Managing Inspections' SOP must:
- Clearly state whom to contact first when the inspector shows up at the site. The facility's quality lead and the site operations lead and their backups should first be notified.
- Describe a mechanism for alerting the entire facility that an inspector is in the building
- Describe how to document FDA requests for information
- Describe how to handle the inspector's request for photographs or videos
- Describe the method to respond to FDA request that is not in agreement with your quality procedures such as entry to cleanroom during operations, hours of operations etc.
It is good to have a team that consists of members from the quality organization and cross-functional groups as part of the inspection readiness team. This team can help in identifying and completing preparation activities and support the inspection throughout.

Predetermine/allocate workspace rooms for inspectors and audit support
It is recommended that the FDA representative workspace be away from heavy traffic areas. The audit support room, also known as the war room should not be located close to the FDA representative's conference room. The war room sometimes becomes busy and inadvertently loud.
Predetermine the SMEs and presenters of protocols deviations will be
It is good to have mock-up sessions and presentations defending SOPs, deviations, trend reports, and validations. This will provide an opportunity for Quality manager to select the right SMEs. Presenting well is a skill that some don't possess. Some get nervous while presenting material or over talk while presenting an issue. Hence, practice sessions are important to the completion of a successful inspection. The presenters should be knowledge, and confident in their presentations.
Identify Records FDA are likely to Audit
Good organization of documents is important to successful completion of inspections. Organize all Regulatory Files by general heading arranged in chronological order (or reverse chronological order). A visual presentation of documents can instill confidence in the inspector that the cGMP documentation practices are implemented and followed. Well-written summaries too go a long way in simplifying the inspection. Inspectors may prefer well-written summaries over bulky documents.
Develop a strategy to justify decisions made
Think through in advance about the hot topics that are more difficult to defend and enlist them. It could be a validation report that has a lot of deviations and discrepancies, or it could be a deviation with a justification that may not seem to be sufficiently robust. Once you enlist such hot topics, develop a strategy to defend the decisions made. Defending needs forethought and practice especially when it comes to articulating data and its conclusions. Practicing is the key.
During the inspection
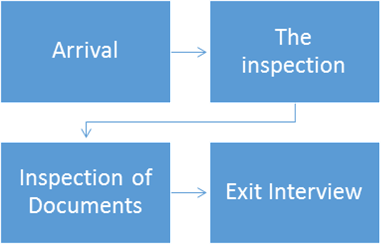
Arrival
- Record the name, date/time. Purpose and escort name if you are using a sign in log and the inspector will not sign in
- Notify the quality head and the operations head and their backups about the arrival of the regulatory inspectors.
- The inspector will present his credentials that they are in order.
- In the opening meeting, obtain information about what type of inspection will be conducted. This is important information to be obtained because the approach 'for cause' inspection is different from the others.
The following table lists the types of inspections and a brief description.
| Inspection type | Brief Description |
|---|---|
| Pre-approval | Performed to ensure
|
| Post -approval |
|
| Surveillance | "Drug Manufacturing Inspections" covering both domestic and international inspections |
| For-cause or directed | Any inspection that is other than a routine inspection. |
Inspection, Inspection documents
- Once the inspector presents the credentials, ensure that the escort walks the inspector to the predetermined conference room that is allocated for the inspector.
- There should be no confidential records in the workspace designated for the FDA inspector(s). At all times, escort the inspector or designee during the inspection and review of documents.
- Inform the entire site that the inspection is in progress.
- The inspection readiness team should begin the audit support room setup plan. Support required will include printers, inspection request log, phones, computers, runners and scribes.
- A brief introductory presentation showing the organizational chart, headcount, hours of operation and facility layout can be made on the first day. Also, inspection will include a facility tour and that is generally on the first day. Provide the inspector a copy of the facility diagram plainly depicting the equipment flow and the personnel. Any housekeeping must be completed before the facility tour begins leaving no chance for the inspector to raise questions about housekeeping procedures.
- The inspector will make a note of the equipment numbers and personal names during the tour. On completion of the tour, this information will be utilized for maintenance of requests, calibration, and training records. Each document request should be logged and sent to the audit support room. Ensure that the inspector does not have to repeatedly ask for the requested documents, provide him/her a reasonable timeline for delivery of the request and deliver as per the timeline.
- Vagueness on the part of the inspector can be a deliberate technique to see what information is given. So on your part, make sure you ask the inspector to clarify. Be honest in answering questions. Take corrective actions if possible, commit only to what can be delivered. DO NOT volunteer information. DO NOT guess or speculate. DO NOT panic. DO NOT sign affidavits.
- Get debriefed of the day's observations from the inspector at the end of each day. This is the time to notice the direction the inspection will take for the next day. It provides the staff with an opportunity to keep the documentation required for the subsequent day.
- Files requested for the purpose of review should be provided to the inspector by the host. The inspector must not access any site records not provided by the host.
- The standard inspection does not include study finances and personnel records.
- The host should set aside time every day to talk with the inspector and be available to answer questions that may arise.
- When documents are copied for inspectors, make a copy to retain or identify the copied document by maintaining an inspection record log.
- Stamp each copy you proved as "Confidential".
- Ensure the requested documents are compiled.
- When documents are copied for inspectors, make a copy to retain or identify the copied document by maintaining an inspection record log.
- Documents that the inspector is not entitled to review or copy: financial, personnel (except for training/qualification records), and internal audits (section 704(a) FDC Act).
- Photographs- If the inspector insists on taking photographs, take duplicates at the same time
Exit Interview
- The Inspector will generally hold an exit interview at the end of the inspection. The escort, the quality head and the operations head and their backups, a representative from Institutional Compliance, and other individuals as appropriate should be informed of the time and place and expect to attend.
- If serious deficiencies have been identified during the inspection, the regional office will send and Inspectional Observations form 483 listing the deficiencies.
- Both the quality head and the investigator will ensure everything is clear.
- Observations, comments, and commitments should be noted in the escort inspection notes
Follow up
- If a 483 is issued, make a written response by including the specifics.
- Was the finding an oversight/one-time occurrence or systematic
- What corrective actions will be taken and why the proposed response will correct the issue.
- Provide a reasonable timeline for the correction
- If the quality head is in disagreement with an observation, respond with facts and verifiable evidence.
- Address every finding point by point.
- Send the reply within two weeks
- Maintain a copy of the final signed response
Additional resources
- Specific course for Laboratory inspection readiness is here.
- Specific course that covers case study derived from actual inspections wherein FDA performed a sponsor site inspection having already audited three of the investigator sites
- 2 day in-person seminar 'Managing Your FDA Inspection: Before, During and After' will cover the factors used by the FDA to schedule inspections. You will learn how to predict what an FDA investigator will do and what they will cover in the inspection. There should be no surprises if you have prepared properly. Firms need to understand the details about inspectional techniques to avoid making new problems for yourself during the inspection. You can save yourself a lot of corporate misery if you know what to do before, during and after an inspection.
Seminar Instructor Casper Uldriks is an "Ex-FDA Official" who has spent 32 years in FDA. He currently trains FDA personnel and counsels clients on wide range of topics, including: FDA inspections; import operations; advertising and promotion; corrective and preventive actions; medical device reporting and corporate reorganization to improve conformance to the FDA's requirements.






