Latin America Market Entry Strategies for Medical Device Companies
The medical device markets in Latin America (LA) is estimated to be worth $ 11 billion and is rapidly growing. How can a medical device company gain fast access to the LA markets? What are the market entry channels, processes, and typical models? This article explains.

The steps involved are shown in the figure below:
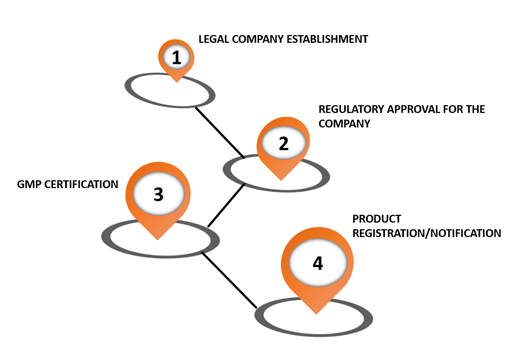
For a detailed understanding of the regulatory compliance, attend the seminar 'Latin America: Regulatory Compliance Requirements for Life Science Products (Focus: Brazil, Mexico, Argentina).'
Argentina, Bolivia. Brazil, Chile, Columbia, Mexico, Paraguay, Uruguay, and Venezuela are the Latin American countries that have a structured regulatory affairs system.
Legal company establishment:
Registration management considerations - Latin America
Distribution
- Varies by Country within Region
- Localized relationships necessary
- Infrastructure only supports Independent Distributorships
- Multiple franchise product responsibility
- Ratio Management - Manage capital/inventory
Competition
- Local competition knockoffs
- Typically "Influential" Surgeon driven/owned
- Little patent protection from Govt. - "Keep money in Country"
- Ratio Management - Manage capital/inventory
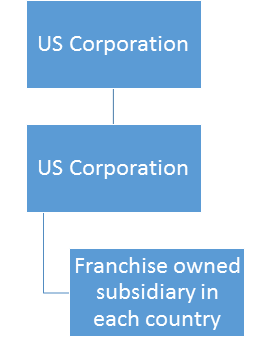
There are 3 ways for the legal establishment of a Company in Latin America.
- Through the establishment of a subsidiary
- Through a distributor that already has the regulatory licenses
- Through an Authorized Representative (AR)
Each of the above ways has their advantages and disadvantages as outlined below:
- Through the establishment of a subsidiary:
- Through a distributor that already has regulatory licenses:
- Through an Authorized Representative:
- Document checklist to register medical devices in Latin America
- Company's working permit
- Free sales certificate (when applicable) Consularization in closest embassy/Consulate of the country where the product will be registered.
- GMP certificate (when applicable)
- Certificate of conformity (For electro-medical devices only)
- Instructions of use, inserts, user manual
- Service/installation manual
- Labeling
- Packing
- The composition of the medical device
- Basic manufacturing flowchart with operations made in each phase
- Validation of sterilization protocol (when applicable)
- Proofs of safety and efficacy
- Additional documents required
- Controls used by the manufacturer during the manufacturing phase.
- All Quality Certificates obtained by the Manufacturer like ISO 9001, 13485, CE Mark, 21 CFR FDA etc.;
- All Quality Certificates obtained by the third party companies that work for Manufacturer like ISO 9001, 13485, 21 CFR FDA, etc., when applicable
- Clinical studies performed with the product itself or similar products to prove the efficacy and safety
- If the prosthesis contains any material originated from cattle then Animal Health Certificates issued by the Veterinary authority of the country of origin of the cattle must be presented
- Extra documents are required for
- Electro-medical equipment
- Condoms and surgical gloves
- In-vitro diagnostic kits
- Orthopedic Prosthesis
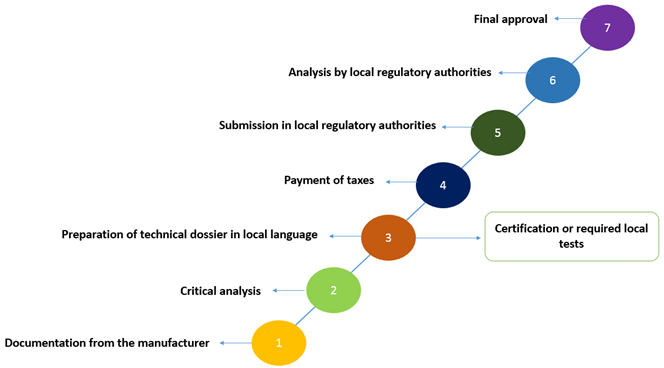
- Steps to medical device registration
- Basic structure of medical devices registration dossier
- Forms according to local legislation tax paid
- Labelling according to local legislation
- Instructions of use/user manual according to local legislation
- Technical report
- Certificate of conformity (for electromedical devices only)
- Proofs of efficacy and safety
- Notarized copy of the free sales certificate* (when applicable)
- Notarized copy of the letter of distribution* (when applicable)
- Local (LATAM) GMP certificate
(*) documents must be authenticated by the consul office) in the closest consulate of the country where the product will be authenticated - A look at Typical Models
Establishing a subsidiary allows the Company to have registrations in its name. It provides the Company the flexibility to appoint Distributors or change Distributors anytime. On the flip side, establishing a subsidiary takes more time. The Company should first obtain the state or city regulatory license and the authorization from the federal regulatory authority before applying for product registration. For medical device Companies, the product registration timeline is between 4 to 6 months and for medical consumables and disposables, the product registration takes about 4 to 9 months.
This is a faster way to enter the market. If the distributor has all the regulatory licenses, this option is a much faster way to enter the market. Many distributors can have the registration of the same product. However, the licenses will be in the name of the distributor(s) and not the foreign Firm. The licenses generally cannot be transferred unless in case of incorporation, fusion or separation of Companies.
If the Authorized Representative (AR) Company has the regulatory licenses, the foreign Company can register the product in the local regulatory agency which, as already stated takes about 4 to 9 months. The foreign Company can change the distributor as necessary any time. Using the AR's registration number, the distributor can import the products. Also, using the AR's registration number, the holder of the product's registration can authorize its local distributors or final consumer (Hospitals, Labs, and Clinics) the product can be imported.
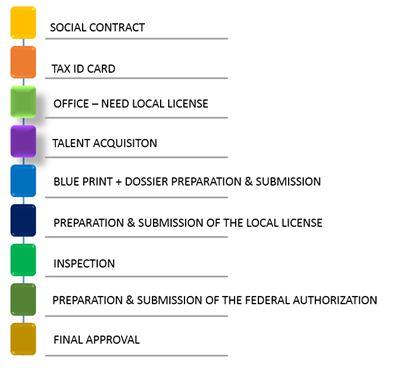
The process to obtain Company approval
Technical drawings of all components with the physical dimensions and tolerances;
Large country models: Italy, Germany, UK, France & Spain
Small country model: Czech Rep, Poland, Turkey...
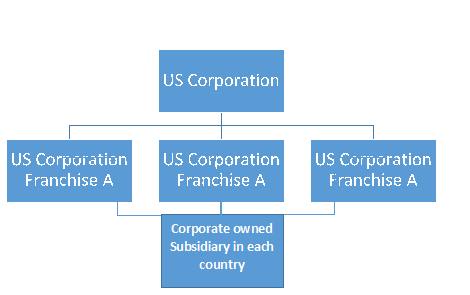
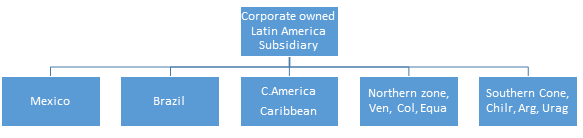
Latin American Model
Attend the seminar 'Latin America: Regulatory Compliance Requirements for Life Science Products (Focus: Brazil, Mexico, Argentina) to understand the structure of the regulatory agencies in Latin America. The seminar will help you successfully overcome local cultural nuances in working with the regulators.
The course director Robert J. Russell, (Bob) has more than 30 years of experience working with FDA, EMA, Healthcare Authorities and Agencies across Latin America, Middle East and Asia / Pacific supporting clients projects in these regions. Licensing, registrations, GMP, DMFs and borderline products are his core competencies.






