Statistical tools for Regulatory Compliance, Pharmaceutical Control and Development
If you are a practitioner in validation and compliance, you must understand and utilize statistical methods for quality engineering whenever possible. Your work provides the basis for future manufacturing and selection of criteria in pharmaceutical process. Documentation of your work is used in regulatory submissions, regulatory audits, change control, and other activities that support products and processes. Important statistical concepts and methods are essential for making objective decisions related to product quality.

This article provides information on statistical methods useful in daily work situations.
FDA's emphasis on the importance of statistical methods
The FDA Process Validation Guidance released in January 2011 emphasized the importance of statistical expertise is emphasized throughout. Starting from pharmaceutical product process design and development through ongoing commercialization, the guidance has transformed process validation from an individual and singular event to an ongoing continuum. A comprehensive approach to process validation is incorporated in the guidance by including modern concepts, quality-by-design (QbD), process analytical technology (PAT), and risk management.
The statistical analysis and models that are relevant for analytical validation methods are described in the guidance. The other processes such as cleaning, packaging, qualifications such as equipment, facilities, utilities, control systems, hybrid systems such as water, heating, ventilation, and air conditioning [HVAC], and quality systems apply the same principles and approach described above. As measurement itself is a process, the statisticians play a crucial part in the evaluation of the measurement process which is important to the continuation of most other work. Consisting of multiple sub-processes and sub-sub-processes and so on, the pharmaceutical processes have the measurement as the foundation of all of these. The higher-order processes can be studied only if the measurement process is evaluated. When variation and uncertainty exists, statistical methods are the tools that are utilized for better risk-based decision-making.
Process validation defined
"Process validation is defined as the collection and evaluation of data, from the process design stage throughout production, which establishes scientific evidence that a process is capable of consistently delivering quality product. Process validation involves a series of activities taking place over the lifecycle of the product and process. This guidance describes the process validation activities in three stages:
- Stage 1 - Process Design: The commercial process is defined during this stage based on knowledge gained through development and scale-up activities.
- Stage 2 - Process Qualification: During this stage, the process design is confirmed as being capable of reproducible commercial manufacturing.
- Stage 3 - Continued Process Verification: Ongoing assurance is gained during routine production that the process remains in a state of control."
"Effective process validation contributes significantly to assuring drug quality. The basic principle of quality assurance is that a drug should be produced that is fit for its intended use. This principle incorporates the understanding that the following conditions exist:
- Quality, safety, and efficacy are designed or built into the product.
- Quality cannot be adequately assured merely by in-process and finished-product inspection or testing.
- Each step of a manufacturing process is controlled to assure that the finished product meets all quality attributes including specifications."
Regulatory requirements and recommendations
Process validation is an enforceable requirement in the pharmaceutical good manufacturing practices (GMPs) for finished product: Emphasis is laid on the recognition of variation and control in the guidance. Aspects of process validation include both sampling and in-process specifications. Both these areas require statistical analyses. Statistical data gained from the sampling plans must provide assurance that the product batches satisfy the predetermined specifications. The application of statistical procedures must determine the in-process limits. FDA's emphasis on the recognition of variation and associated control is seen from the list of recommendations the guidance provides. A team approach to process validation and the demonstration of expertise in statistics is also recommended.
Although activities in each stage of the process validation are described in the guidance, in practice some activities in different stages might overlap.
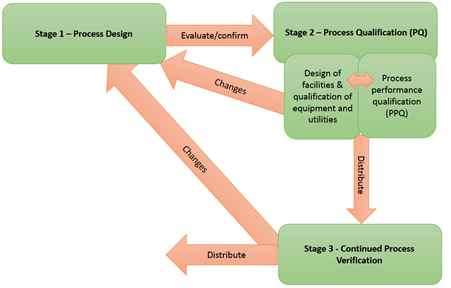
Stage 1- Process Design
Stage 1 recommendations address development activities that will ultimately be reflected in the master production record and control records. It includes:
- Higher level of sampling
- Additional testing
- Greater scrutiny of process performance
- Above could be extended in certain cases e.g. complexity of process, production volume
- Manufacture under normal conditions using routine personnel
The goal of Stage 1 is "To design a process suitable for routine commercial manufacturing that can consistently deliver a product that meets its quality attributes."
Stage 2- Process Qualification
- This stage consists of the performance of the commercial process by means of conformance lots
- It confirms work of Stage 1 Process Design
- Demonstrates that the planned manufacturing process is capable of reproducible commercial manufacture
- The acceptability of the developed formulation and process must be demonstrated through increased testing
- Discusses design of facility, utilities, and equipment, Process Performance Qualification (PPQ), the PPQ protocol, and PPQ protocol execution and report in Stage 2 all of have a direct connection with specific process validation.
- Must be based on sound science and experience as developed in stage 1 studies and activities.
The goal of stage 2 is to show that the process is reproducible and will consistently deliver quality products.
Stage 3 - Continued Process Verification
The guidance clearly demonstrates scope, objectives, and criticality of data analysis and statistical treatment of data in Stage 3. Of special interest is the FDA's recommendations with regard to expertise in statistics.
"An ongoing program to collect and analyze product and process data that relate to product quality must be established. The data collected should include relevant process trends and quality of incoming materials or components, in-process materials, and finished products. The data should be statistically trended and reviewed by trained personnel. The information collected should verify that the quality attributes are being appropriately controlled throughout the process.
We recommend that a statistician with adequate training in statistical process control techniques develop the data collection plan and statistical methods and procedure used in measuring and evaluating process stability and process capability. Procedures should describe how trending and calculations are to be performed and should guard against overreaction to individual events as well as against failure to detect unintended process variability. Production data should be collected to evaluate process stability and capability. The quality unit should review this information. If properly carried out, these efforts can identify variability in the process and/or signal potential process improvements.
"Many tools and techniques, some statistical and others more qualitative, can be used to detect variation, characterize it, and determine the root cause. We recommend that the manufacturer use quantitative statistical methods whenever feasible."
The goal of the third validation stage is continual assurance that the process remains in a state of control (the validated state) during commercial manufacture.
Regulatory expectations with regard to variation, control, and statistics
"A successful validation program depends upon information and knowledge from product and process development. This knowledge and understanding is the basis for establishing an approach to control of the manufacturing process that result in products with the desired quality attributes. Manufacturers should:
- Understanding sources of variation
- Detect the presence and degrees of variation
- Understand the impact of variation in the process and ultimately on product attributes
- Control the variation in a manner commensurate with the risk it represents in the process and product.
Each manufacturer should judge whether it has gained sufficient understanding to provide a high degree of assurance in the manufacturing process to justify commercial distribution of the product. Focusing exclusively on qualification efforts with also understanding the manufacturing process and associated variation may not lead to adequate assurance of quality. After establishing and confirming the process, manufacturers must maintain the process in a state of control over the life of the process, even as materials, equipment, production environment, personnel, and manufacturing procedures change.
Manufacturers should use ongoing programs to collect and analyze product and process data to evaluate the state of control of the process. These programs may identify process or product problems or opportunities for process improvements that can be evaluated and implemented through some the activities described in Stages 1 and 2.
Manufacturers of legacy products can take advantage of the knowledge gained from the original process development and qualification work as well as manufacturing experience to continually improve their processes. Implementation of the recommendations in this guidance for legacy product and processes would likely begin with the activities described in Stage 3."
FAQs on Statistical tools for Regulatory Compliance
1. How does the FDA view statistical expertise in validation?
The FDA emphasizes that statistics are critical at every stage of process validation. From initial design to ongoing production, FDA guidance encourages the use of statistical analysis to detect variability, demonstrate control, and make data-driven decisions. They recommend involving trained statisticians to design data collection plans, analyze trends, and evaluate process stability.
2. How do the three stages of process validation differ in terms of statistical requirements?
- Stage 1 (Design): Collect extensive data on process performance, test under normal conditions, and scrutinize variations.
- Stage 2 (Qualification): Demonstrate reproducibility through controlled production lots and statistical evaluation of performance.
- Stage 3 (Continued Verification): Use ongoing statistical monitoring of process trends to maintain control and identify improvement opportunities.
Each stage progressively builds confidence that the process is capable and remains reliable.
3. Can statistical methods help with legacy products?
Absolutely. Even for legacy products, statistical tools can help manufacturers understand historical variation, assess process stability, and identify areas for improvement. Implementing Stage 3 activities—ongoing data collection and trend analysis—can enhance control and optimize the process over time.
4. How do statistics support regulatory submissions and audits?
Regulators require evidence that your process is under control and capable of consistently producing quality products. Statistical data—like trend analyses, control charts, and capability studies—provide objective, defensible proof. They show regulators that your decisions are data-driven, not just based on observation or guesswork.
5. How do statistical methods improve risk-based decision-making?
By quantifying variation and understanding its impact on product quality, statistics help prioritize risks. For example, if a particular step shows high variability, you can implement tighter controls, additional testing, or process improvements. Decisions backed by statistical evidence reduce uncertainty and increase confidence in quality outcomes.






