The U.S. Food and Drug Administration (FDA) Import Requirements
All FDA regulated products are electronically screened before they enter the U.S. If it appears that the product violates the U.S laws, FDA may refuse entry. FDA's screening program, PREDICT, and U.S. Custom's ACE program requires greater attention to details. Filing incorrect entry data in ACE can attract penalties and greater scrutiny for data verification. Once an error occurs, getting it resolved can be frustrating. The legal Authorities FDA and U.S. Customs and Border Patrol (CBP) rely on each other's import requirements to determine the entry of the product. Understanding the requirements can help importers avoid costly errors and delays.

Submitting Entries of FDA-regulated Products
The first step to any importation process begins with the import broker notifying the CBP service of the entry. CBP collects tariffs, taxes, and fees for the products.
- Establish an account with CBP
- Work with an import broker who is familiar with FDA imports and ideally with your type of product. To find a port of entry and customs trained brokers, click here.
- Use proper documentation with each shipment
- Use a CBP commercial invoice
- Keep complete and accurate records
- Establish orderly import operations to manage the regulatory matters
Meeting FDA Regulatory Requirements
FDA import program operates under the authority of the Federal Food, Drug, and Cosmetic Act, as amended (FDCA) (21 U.S.C. Part 381). The FDA's legal authority is broadened with a number of other laws it relates to:
Examples include:
- Fair Packaging and Labeling Act
- Radiation Control for Health and Safety Act
- Public Health Service Act
- Comprehensive Smoking Tobacco Health and Education Act
- FDA Food Safety Act
It is mandatory that the initial importer establishment must be registered with FDA. The initial importer along with the U.S. import broker is responsible for communicating with the legal authorities.
The product is deemed to be in violation when it is in some way adulterated or misbranded:
If the product appears to be in violation of the FDA law, the FDA notifies the initial importer or the consignee of the specific reason or misbranding charge.
An adulteration charge will identify a problem with the product itself. Examples include:
- The product is contaminated with bacteria
- Consists in whole or in part of any filthy, putrid or decomposed substance
- Prepared or stored in contaminated conditions
- The manufacturing methods and controls are not compliant with cGMP
- Falls below its strength, quality or purity it claims to have
Examples of misbranding charges include:
- False or misleading labeling in any in particular
- False or misleading advertising
- Missing information on the label
- Inadequate directions for use
- Unregistered establishment
Import Information required by the FDA:
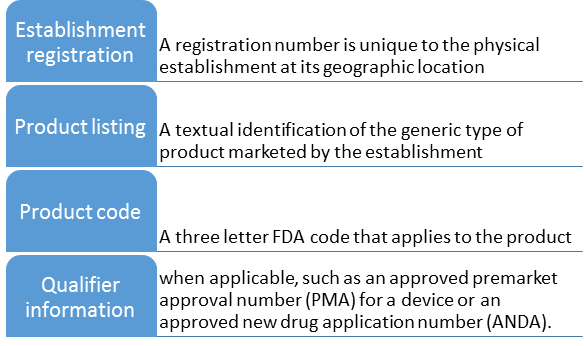
Initial FDA Import Process
To begin the FDA import process, the establishment registration fee must be paid. If all the requirements are in compliance, the FDA will issue an email confirmation that the registration is complete.
If the information is incomplete or inadequate, FDA's import computer program will automatically detain the shipment. Each piece of information should be consistent with the rest of the information requirements. If the product is detained, the importer and broker should resolve it immediately with the District's import officer who is responsible for the port of entry. Repeated detentions can be costly. It can be frustrating to the importer, his broker, CBP and FDA officers. Besides, there are long-term consequences.
Documents Required by FDA
- Bill of Lading (BOL)
- Airway Bill (AWB), invoice
- Purchase order
Where applicable the importer should also provide: FDA the commodity-specific certifications (USDA permits, Impact Resistance test results, etc.), packing list/growers list, copies of labeling, documentation stating who the actual manufacturer is, documentation explaining why articles classified as U.S. Goods Returned are being returned, certificate of analysis, intended use statement or End-Use Statement, and other related documentation as requested. CBP forms 3461 and/or 7501 should also be provided to the FDA for manual (Non-ABI) entries.
The importer, entry filer, or other responsible parties can submit the documents to (ITACS) or use and find the import office contact page to determine the local import division email address, postal address, and/or FAX number to submit documents outside of ITACS.
The Accuracy of Information is critical - CBP and FDA Joint Import Computer programs
The FDA and (CBP) rely on the following computer programs to expedite the import process. Understanding how and when to use these systems can improve net profit from even a single shipment.
Automated Commercial Environment Program (ACE)
ACE is used to evaluate compliance and monitor daily data operations for CBP and FDA required information. For detailed information on how to get started with the ACE program click here .
FDA's Computerized Information Screening Program (PREDICT)
PREDICT is FDA's automated screening program that identifies, sorts and analyzes product information. The program issues a Notice of Action (NOA) to indicate the shipment "may proceed" if the initial required information is ok. The PREDICT software program may randomly select shipments to verify the accuracy of required information. If the information does not appear to be correct, the entry information will be reviewed manually. The information for each line item will be verified. This may cause a delay.
FDA's OASIS Program
It is the data collection of import operations for tracking purposes. The database is used to generate FDA's Import Refusal Report (IRR). The information is published monthly as the FDA Import Alert. This program targets products with a higher probability of not meeting FDA requirements.
Affirmation of Compliance (AOC)
The AOC is a program for the submission of information that can expedite the CBP import clearance process.
Automated Broker Interface (ABI)
Brokers use the Automated Broker Interface (ABI) to submit required information electronically.
To understand the New Import Program for 2018 2-Day In-Person Seminar. The speaker Casper (Cap) Uldriks brings over 32 years of experience from the FDA.






