By using this site you agree to our use of cookies. Please refer to our privacy policy for more information. Close
Receipt and Storage of cGMP Controlled Raw Materials Regulations and Best Practices
- By: Staff Editor
- Date: July 23, 2018

Receipt and Storage of cGMP Controlled Raw Materials Regulations and Best Practices
Manufactures of drugs who lack a defined raw material processing have received FDA 483s and lost several batches of products. Designing the Receipt, Handling and Processing, Specification, Inventory Tracking, and Qualification of cGMP Controlled Raw Materials is critical to Life sciences personnel to gain drug approvals.
Steps involved From Receipt through Release for Use of cGMP Raw Materials

This article specifies regulations and briefly discusses the first two steps ‘Receipt and Storage’.
Regulations:
- 21 CFR 211.80 explains what general requirements of CGMP Materials are. For guidance, please refer ICH Q9: Quality Risk Management, and ICH Q8(R2): Pharmaceutical Development.
- 21 CFR 211.82 explains what needs to be done upon receipt and before acceptance of containers. For guidance please refer ICH Q10: Pharmaceutical Quality System and Q&A – CGMP, GGP Level 2 Guidance – Control of Components and Drug Product Containers and Closures
- Storage Guidance Q7 A(7.4)
Receipt of Raw materials:
Upon receipt, the inspection of incoming raw materials is an essential step. Inspection ensures that the correct raw material which meets the quality specifications has been received. It helps in maintaining safety, minimizing wasted time, material cost and delayed shipments if the specifications are not met.
Sampling Strategy 
Storage in controlled conditions
Storage in controlled conditions requires appropriate locations such as warehouses, and quality control areas. Storage also requires compliance during receiving and transferring materials. Quality equipment such as freezers and refrigerators too play a major part in storage control.
A partial list of the good storage practices is given below: 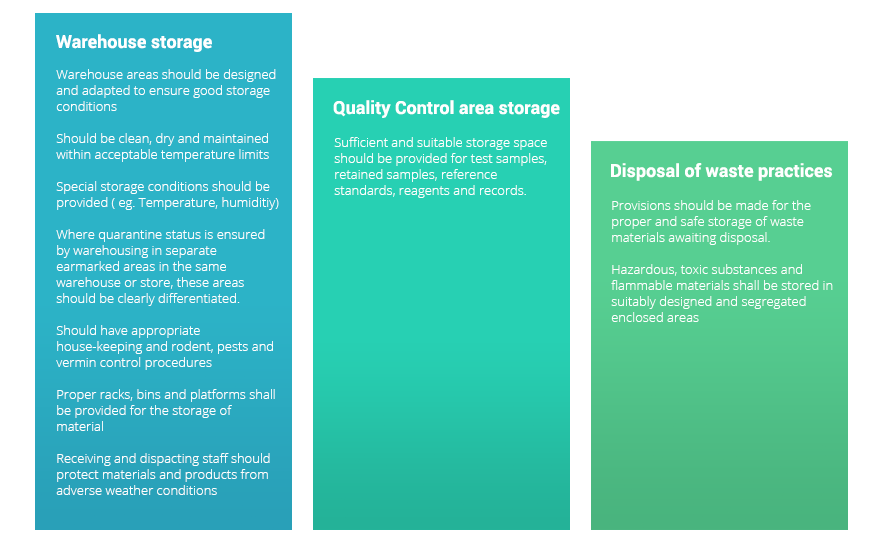
The practices and processes set forth in this general information article apply to the receipt and storage of cGMP raw material control. There are five more steps that require understanding of the cGMPs raw materials best practices. You may wish to attend the webinar that covers these steps in detail. Click here to enroll.
The evolving global processes and products requiring special environmental controls highlights the need for continuous learning on the part of professionals in the life sciences industry.
To stay current, to be ready for the change as new solutions evolve, to develop strategies for the best practices, controls, and procedures, browse through our list of webinars.
Compliance Trainings

By - Jeff Kasoff
On Demand Access Anytime

By - David Nettleton
Live April 02, 2025

By - T.C Soli
On Demand Access Anytime

By - Jim Polarine
On Demand Access Anytime
Compliance Standards
- Add to Cart
- Add to Cart
- Add to Cart
- Add to Cart
- Add to Cart
- Add to Cart
- Add to Cart
- Add to Cart
-
By: Miles HutchinsonAdd to CartPrice: $249
- Add to Cart
- Add to Cart
- Add to Cart
- Add to Cart
- Add to Cart
- Add to Cart
-
Add to CartSan Francisco, CA | Aug 6-7, 2020
-
Add to CartVirtual Seminar | Jul 16-17, 2020
-
Add to CartVirtual Seminar | Jun 18-19, 2020
-
Add to CartLos Angeles, CA | Aug 20-21, 2020
-
Add to CartVirtual Seminar | Jul 16-17, 2020
-
Add to CartVirtual Seminar | Jun 25-26, 2020
-
Add to CartVirtual Seminar | Jun 10, 2020
-
Add to CartVirtual Seminar | Jun 3-4, 2020
-
Add to CartVirtual Seminar | Jul 6-7, 2020
-
Add to CartSan Francisco, CA | Oct 22-23, 2020
-
Add to CartVirtual Seminar | Jul 9-10, 2020
-
Add to CartVirtual Seminar | Jun 3-4, 2020
-
Add to CartVirtual Seminar | June 3-4, 2020
-
Add to CartMiami, FL | Jul 29-31, 2020
-
Add to CartVirtual Seminar | Jun 17, 2020
-
Provider: ANSIAdd to CartPrice: $142
- Add to Cart
- Add to Cart
- Add to Cart
-
Provider: ANSIAdd to CartPrice: $120
-
Provider: ANSIAdd to CartPrice: $250
-
Provider: SEPTAdd to CartPrice: $299
- Add to Cart
-
Provider: Quality-Control-PlanAdd to CartPrice: $37
- Add to Cart
-
Provider: At-PQCAdd to CartPrice: $397
- Add to Cart
- Add to Cart
- Add to Cart
- Add to Cart








